Sony announced in July 2011 that it will use tin (Sn) anode materials to increase the capacity of lithium-ion rechargeable batteries. The battery unit of "18650" size (18mm diameter x 65mm height) was developed this time, with a capacity of up to 3.5Ah. Compared with the company's 2.8Ah original product put into production in 2010, the capacity has increased by 25% (Figure 1). The volumetric energy density is 723 Wh/L. The weight was 53.5 g and the weight energy density was 226 Wh/kg. The charging voltage is 4.3V. The supply is scheduled to begin in 2011.
This article refers to the address: http://
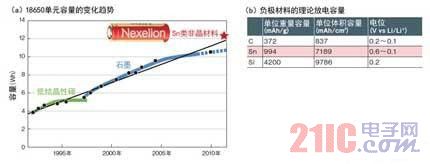
Figure 1: Increase capacity by 25% by using Sn negative
Sony has developed a lithium-ion rechargeable battery "Nexelion" (a) that uses a Sn-based negative electrode material to increase the cell capacity by 25%. Previously, graphite anode materials have been used to increase capacity, but capacity growth rates have recently slowed. Sn has a theoretical capacity (b) that is much larger than that of ordinary negative electrode material graphite.
In fact, this is not the first time Sony has made a lithium-ion rechargeable battery with a negative anode for commercialization. Since 2005, the company has sold "14430" size battery cells with a diameter of 14mm and a height of 43mm for cameras. This time, the 18650 size product will be mass-produced for laptops larger than the 14430.
Amorphization of Sn-based materials
In order to achieve a high capacity of 3.5Ah for the 18650-size battery unit, "only the negative electrode material can be changed" (Sony Hirohiro, Director of Commodity Design Division 1 of the 1st Business Unit of Sony Energy Device LI). Sony increased its capacity by changing the anode material from the original low crystalline carbon (hard carbon) to graphite before and after 1997.
After that, the capacity was increased to about 2.5 times by the improvement of the positive electrode material, but the capacity increase rate has been decreasing recently. As a result, Sony decided to change the anode material of the 18650 unit again after about 14 years, using Sn-based materials.
Sn and silicon (Si) have nearly 10 times the theoretical capacity compared to the current mainstream anode material graphite. However, the expansion and contraction of the negative electrode during charge and discharge destroy the crystal structure, so the charge and discharge cycle life is short. This time Sony solved this problem by amorphization of Sn-based materials (Figure 2). By amorphizing various elements such as Sn, cobalt (Co), and carbon at the nanometer level, the company suppresses the shape change of particles during charge and discharge, thereby improving the charge and discharge cycle life of the battery cells.
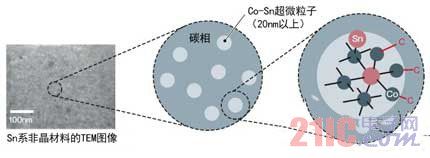
Figure 2: Construction of Sn-type amorphous Co-Sn ultrafine particles
The negative electrode material of the novel lithium ion secondary battery is constructed such that ultrafine particles composed of a Co-Sn alloy are distributed in the carbon phase. Co forms carbides with carbon and combines them.
High capacity also ensures safety
The lithium-ion rechargeable battery developed this time also improved the positive electrode and the diaphragm. The positive electrode material was changed from a ternary (Li(Ni-Co-Mn)O2) material capable of achieving both capacity and safety to lithium cobaltate (LiCoO2). LiCoO2's Co material is expensive and thermal stability is at a disadvantage, so the positive electrode material has been changing to the ternary class, but Sony has reversed the trend and adopted the higher capacity LiCoO2.
LiCoO2 will increase in temperature when an abnormality such as an internal short circuit occurs. In this case, oxygen is generated, and oxygen may be combusted after reacting with the organic electrolyte. Therefore, Sony "treated 0.1 to 1 μm thick on the surface of LiCoO2 particles" (upper hole). According to the company, this method reduces the reaction of oxygen with organic electrolytes.
In the separator, in order to prevent an internal short circuit from occurring, a metal oxide ceramic layer having a thickness of several μm was formed on both surfaces of the polyolefin microporous film (Fig. 3). The ceramic layer has a three-dimensional structure, and metal oxide particles having a diameter of less than 1 μm are connected to the resin as a binder in a mesh shape. When an abnormality occurs, the resin net having high flexibility and adhesion can cause an insulator, that is, a metal oxide, to adhere around the foreign matter, thereby restricting the passage of current.
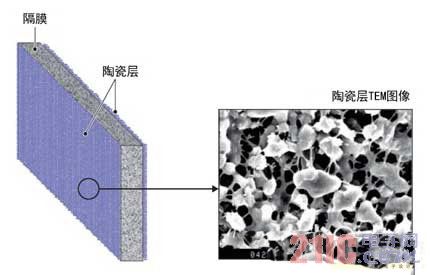
Figure 3: Improving safety through three-dimensional construction of ceramic layers
A three-dimensional structural ceramic layer is formed on both sides of the diaphragm. Once the metal foreign matter is mixed and the diaphragm is damaged, the ceramic layer is transferred to the surface of the metal foreign matter to prevent large current from passing.
Of course, there are still problems with the new unit. For example, after repeated charge and discharge 300 times, the capacity will drop to about 80% of the original. After repeated charging and discharging for 500 times, the capacity will drop to about 60%, and it will barely reach the minimum standard for notebook computers. In order to expand sales in the future, it is necessary to increase the charge and discharge cycle of the battery unit.
PTFE fabric have high performance in smooth and high-glossy coating, which has great release, highest dielectric strength and chemical-resistant characteristic as well. Its outstanding high temperature resistance, ranging from -70°C to +260°C, made it suitale for high temperature situations as heat press machines.
Applications:
- belting for food industry
- Non Stick Baking Liner or oven liner
- electrical insulation
- industrial processing
High Temperature Resistant PTFE Fabric
High Temperature Cloth,High Temperature Fabrics,High Temp Fabric,High Temperature Resistant PTFE Fabric
TAIZHOU YAXING PLASTIC INDUSTRY CO., LTD , https://www.yaxingptfe.com